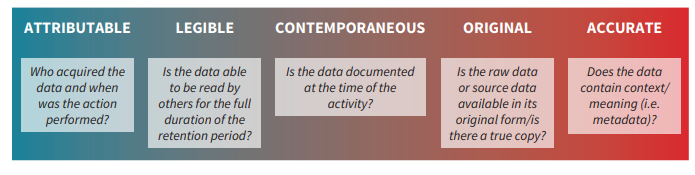
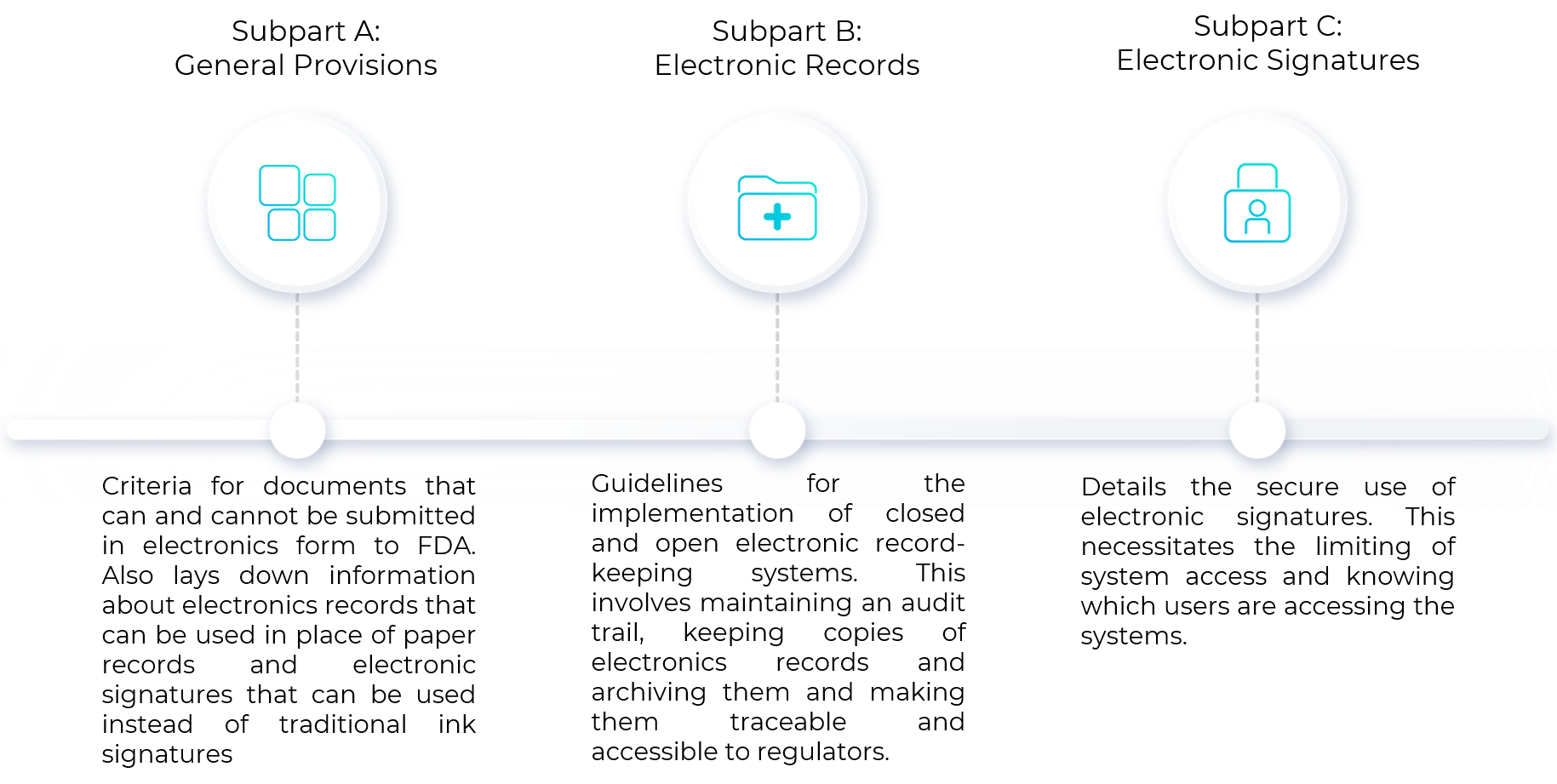
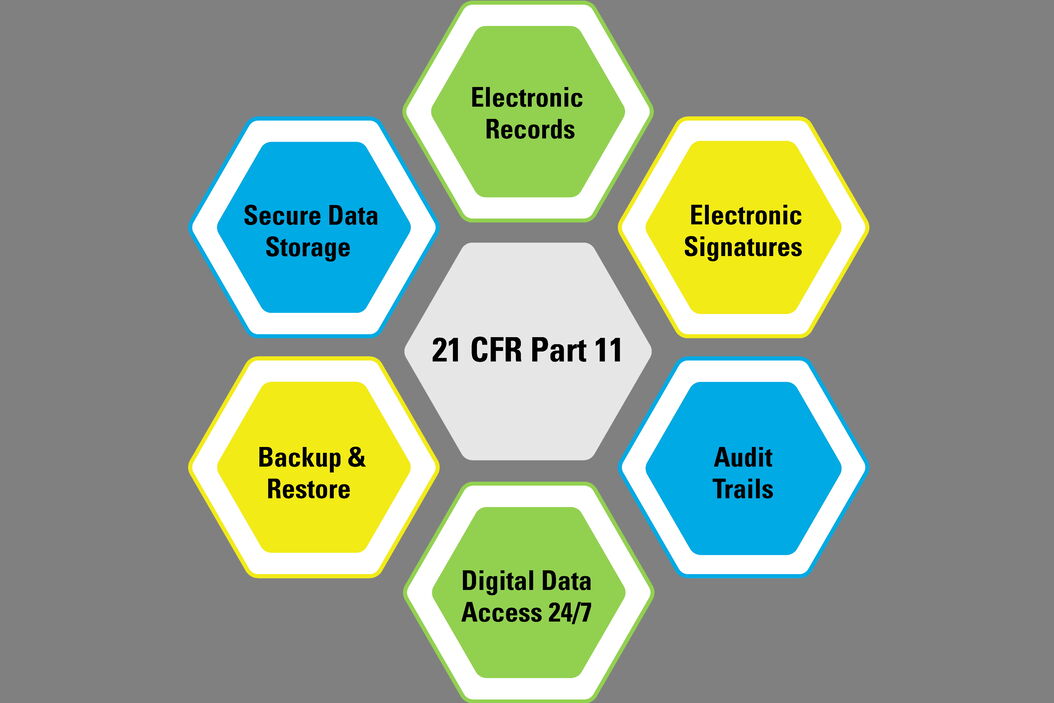
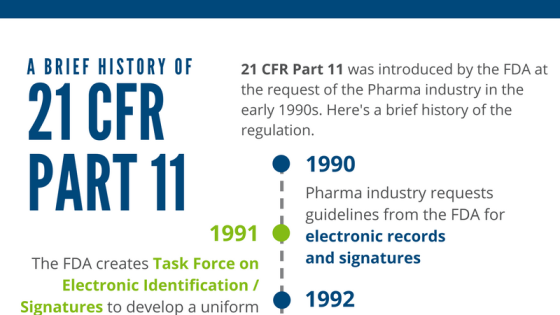
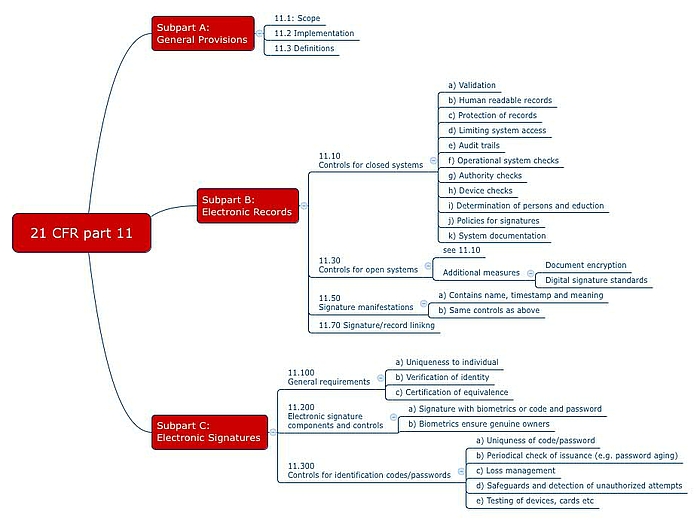
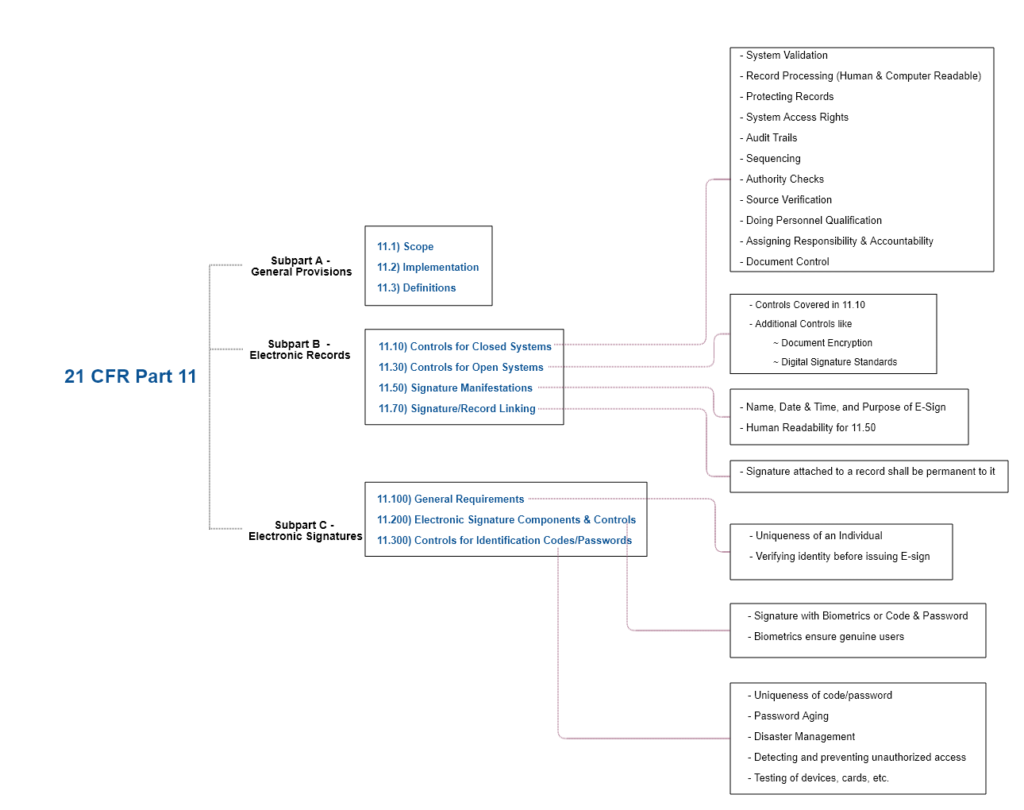
21 CFR Part 11 : Electronic Signature & LMS compliance - DOKEOS - LMS & E-learning Suite for growing companies

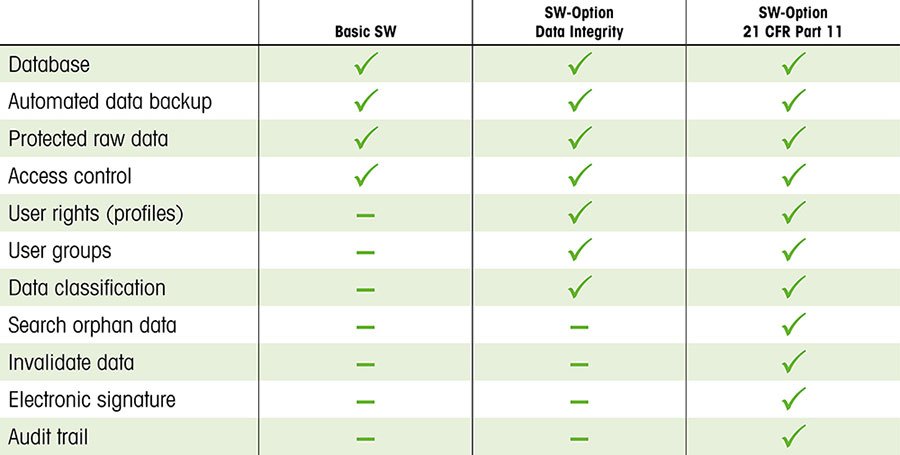
Clarifying and Meeting the Requirements of 21 CFR Part 11 and Data Integrity for Dissolution Testing | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology