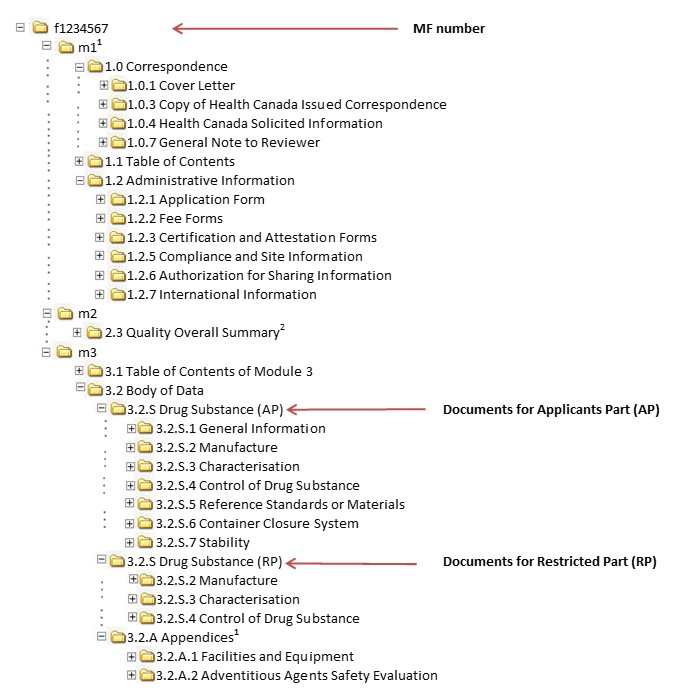
A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S) | PharmaTutor

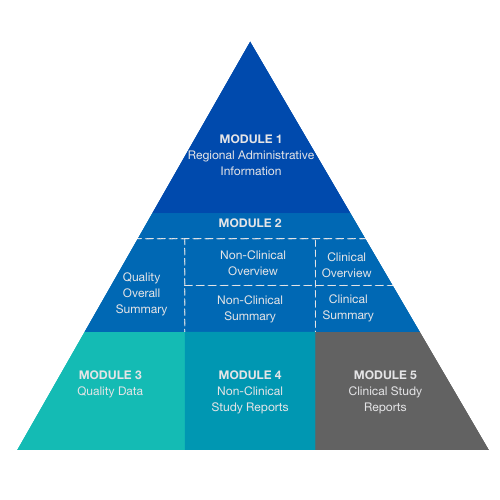
SUB04: Preparing Submissions in the Common Technical Document (CTD) Format | Zenosis – Learning for Life

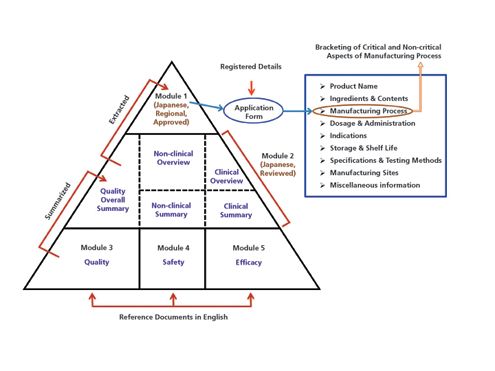
Polifarm doo Beograd - Structure of Common Technical Document (CTD), a set of specification for application dossier for registration of medicinal products | Facebook
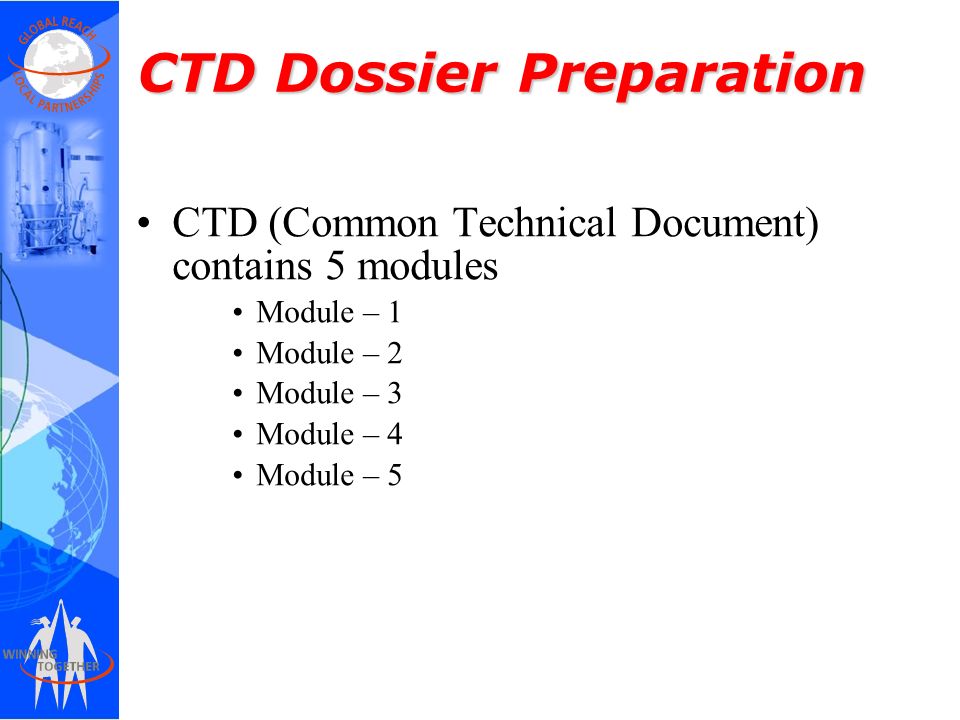
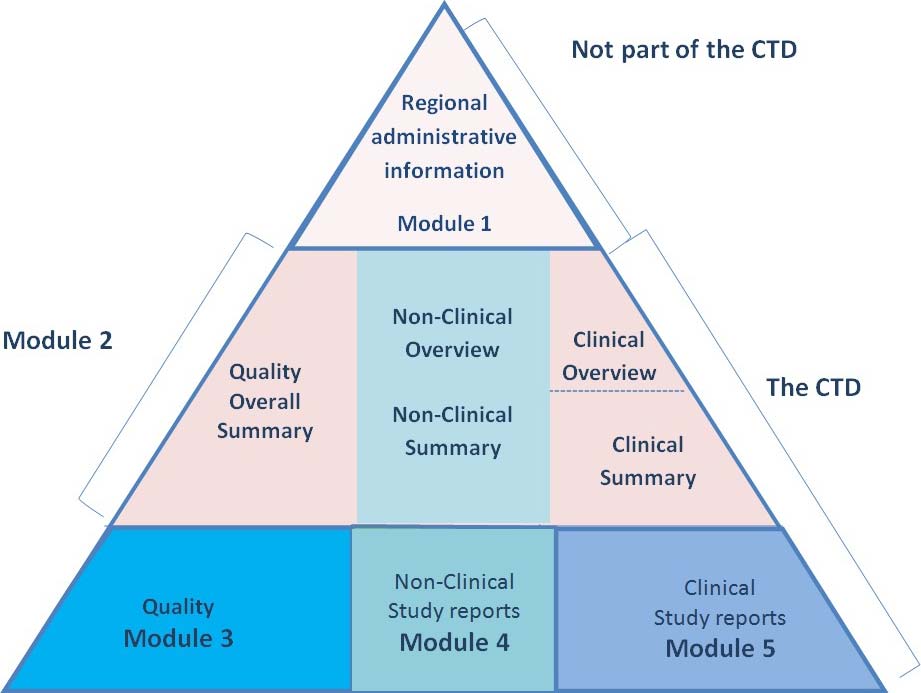
ELECTRONIC COMMON TECHNICAL DOCUMENT (eCTD): A REVIEW OF HISTORY, BENEFITS OF IMPLEMENTING, CHALLENGES, MODULES, RISKS INVOLVED


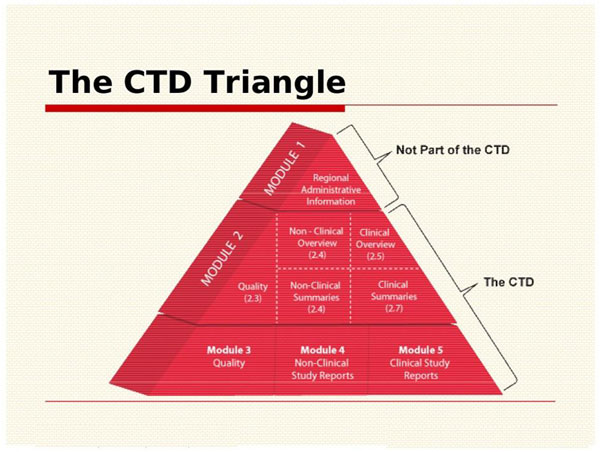
![Common technical document triangle [7] | Download Scientific Diagram Common technical document triangle [7] | Download Scientific Diagram](https://www.researchgate.net/publication/320372658/figure/fig1/AS:550839028203520@1508341666076/Common-technical-document-triangle-7.png)