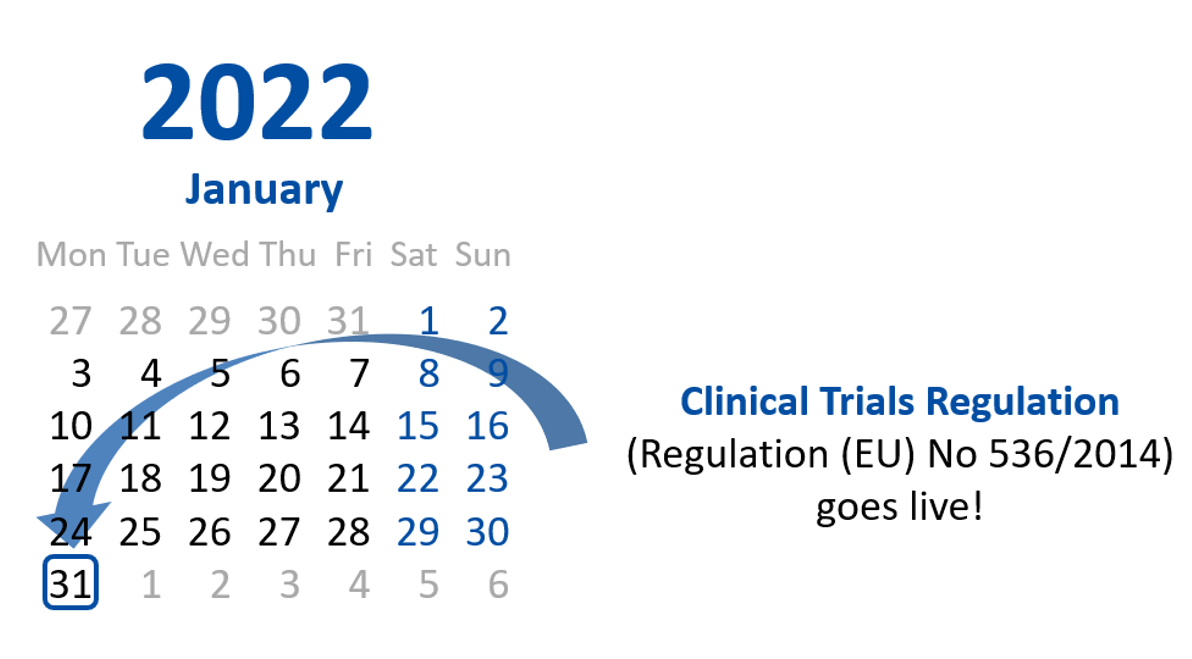
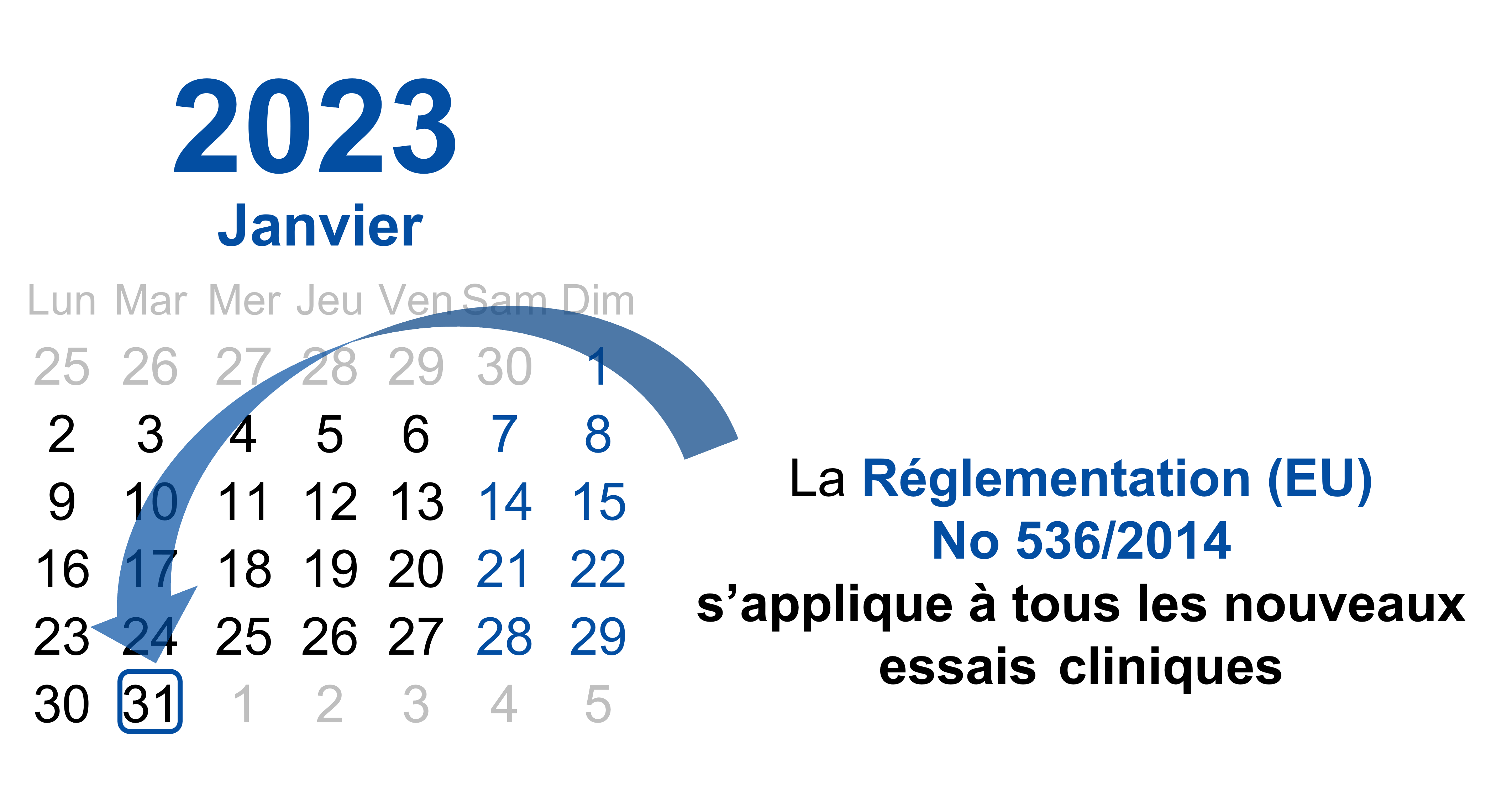
▻B REGULATION (EU) No 536/2014 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 April 2014 on clinical trials on medicinal
April 2022 The rules governing medicinal products in the European Union VOLUME 10 - Guidance documents applying to clinical tria

EU Clinical Trial Regulation 536/2014potential timeline. EoT end of... | Download Scientific Diagram
Questions and answers CTR information session on 23 September 2021 ASMF Active Substance Master File AoR Acknowledgement of rece