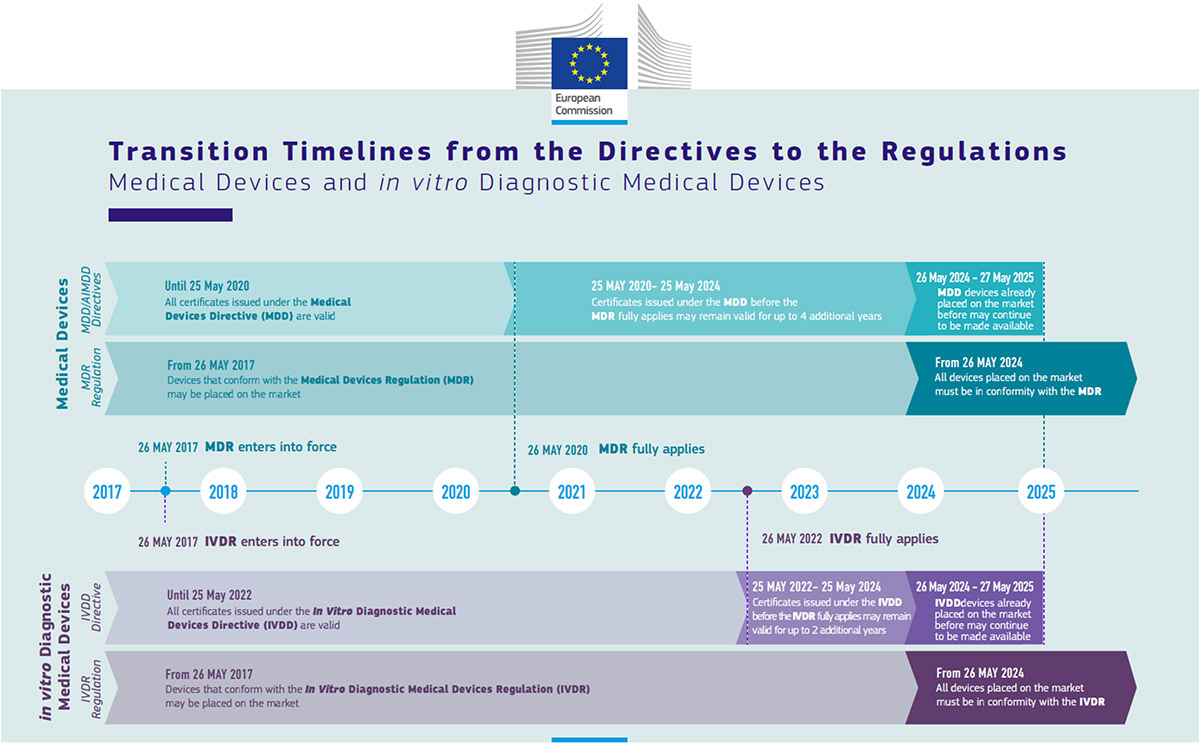
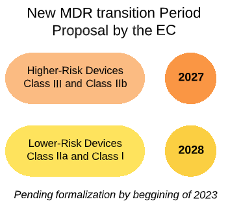
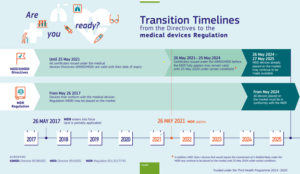
Publicado por @EU_Health el documento de preguntas y respuestas con respecto al Reglamento 2023/607 de modificación plazos transitorios reglamentos MDR e IVDR | Red de Tecnologías Sanitarias y Productos Sanitarios

Formación «2304 – REGLAMENTOS MDR/IVDR y Real Decreto P.S. a 1 y 2 años de su fecha de Aplicación» – 26 MAYO 2023 | Red de Tecnologías Sanitarias y Productos Sanitarios