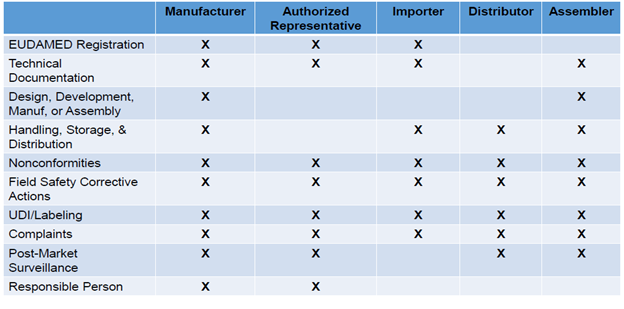
![ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services](https://medidee.com/wp-content/uploads/2022/08/Technical-Documentation-Infographic.png)
ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services
![ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services](https://medidee.com/wp-content/uploads/2022/06/Mdd-Webinars-3-1.png)
ARTICLE] Why You Need To Review Your Technical Documentation NOW (And 8 Pitfalls to Avoid at all Costs) - Medidee Services
MDCG: nueva MDCG 2022-12 Guia de practicas administrativas y soluciones técnicas alternativas hasta que EUDAMED