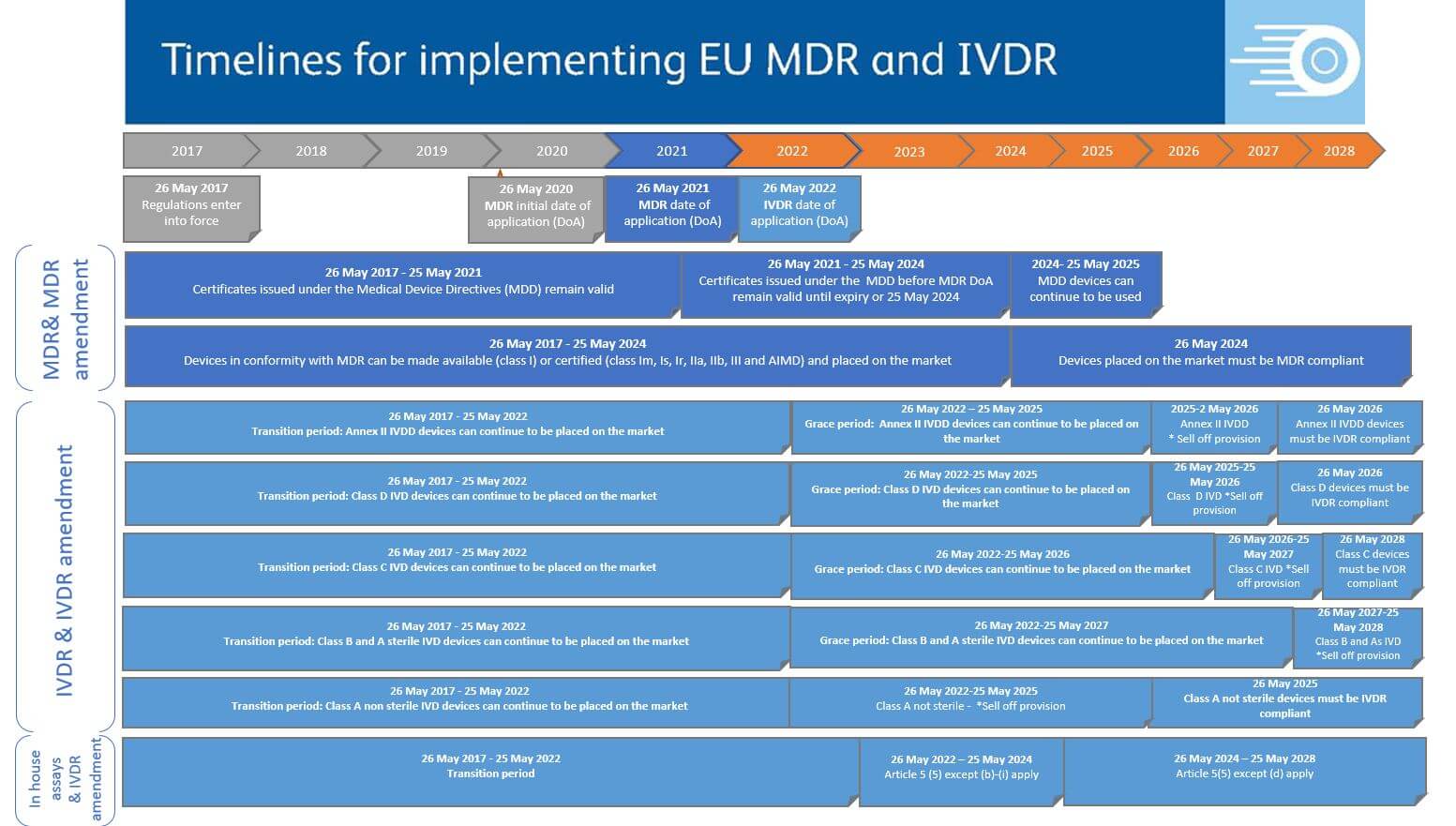
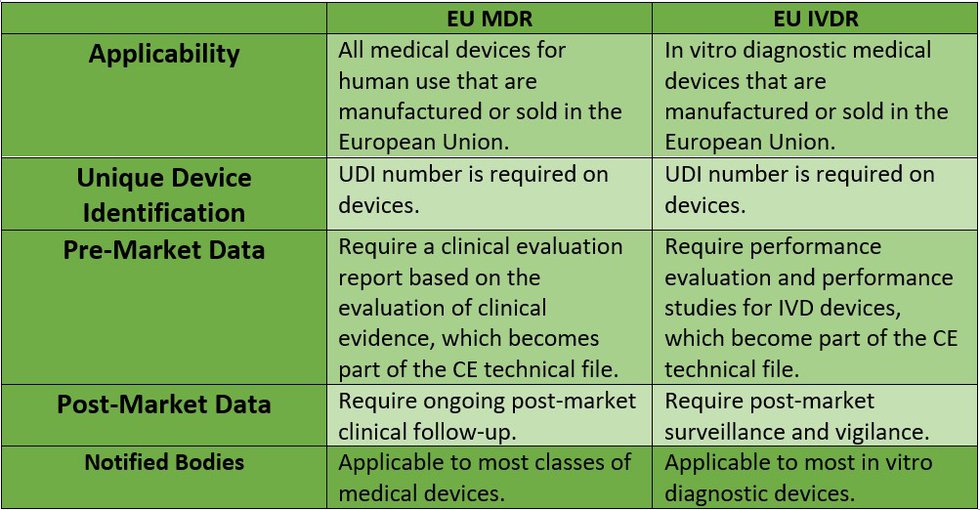
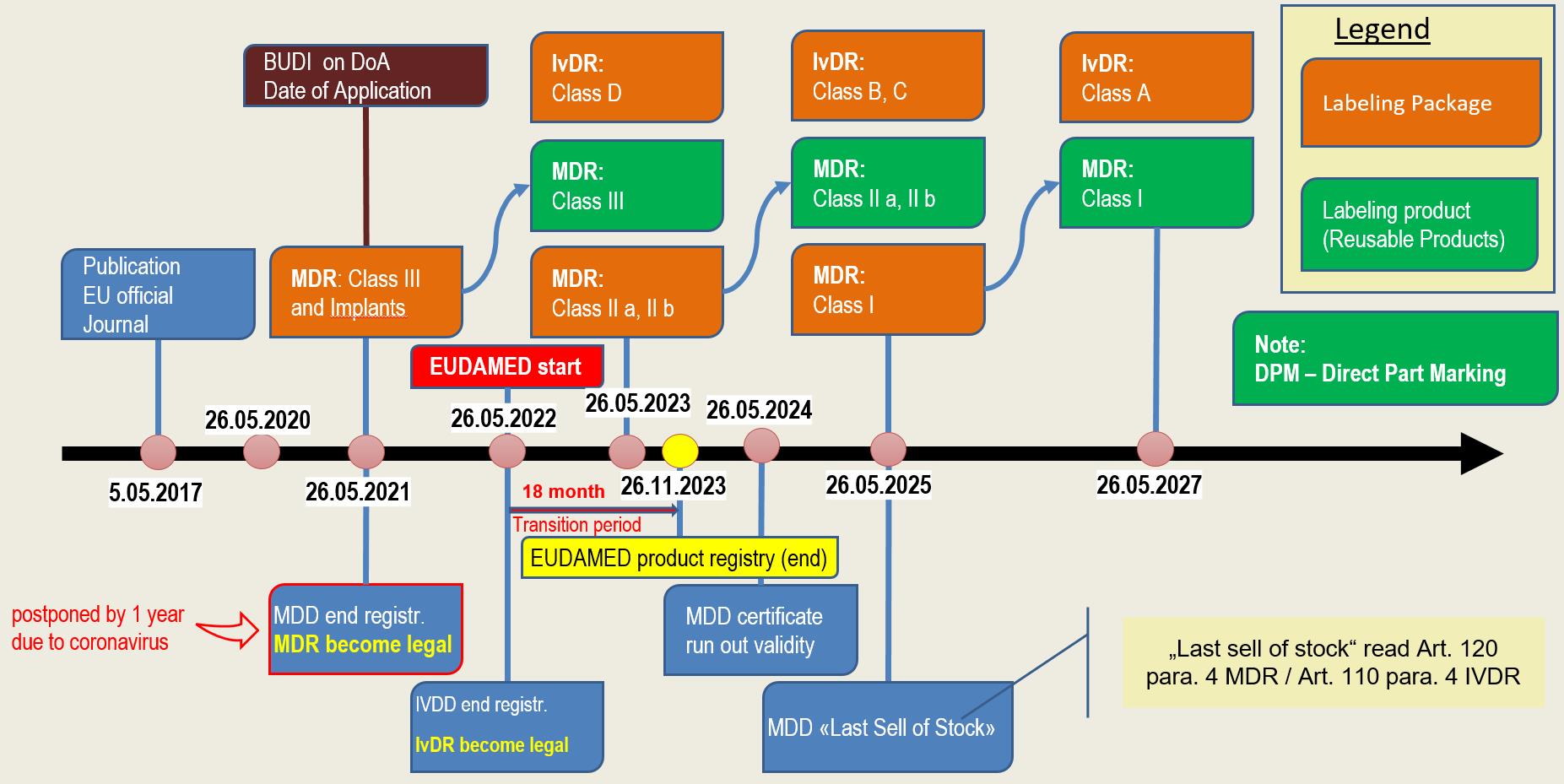
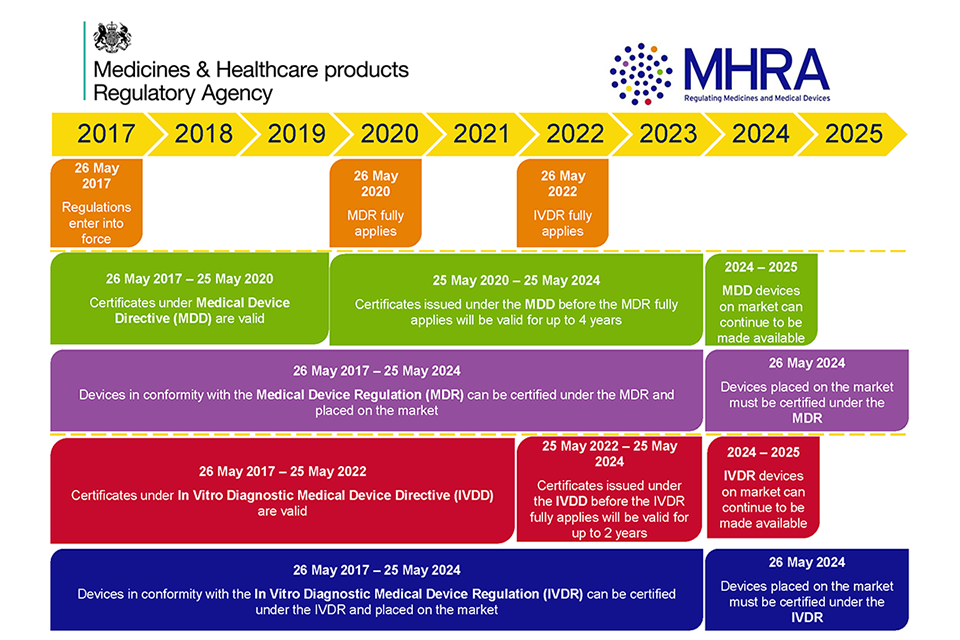
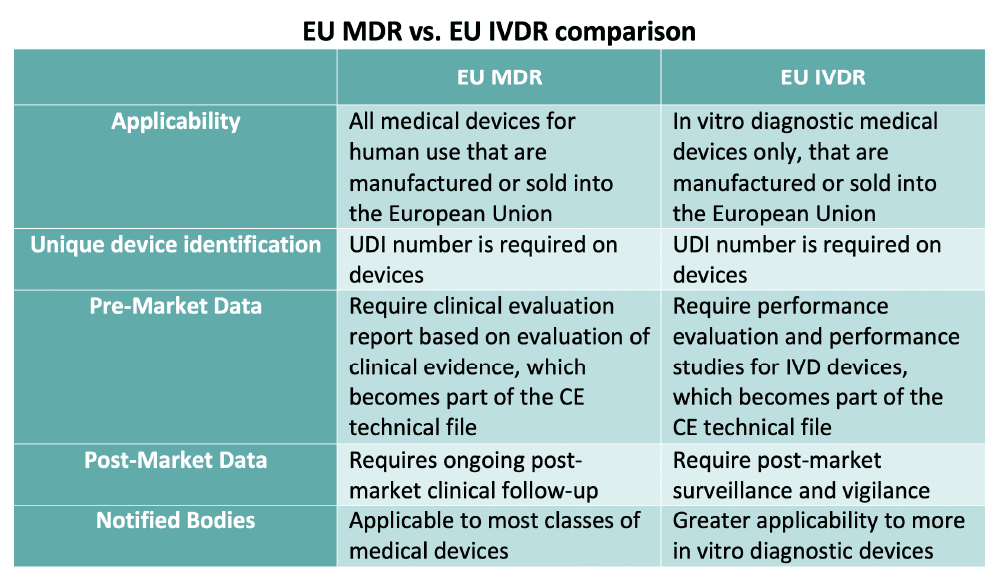
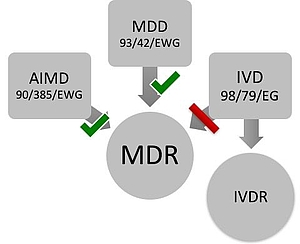
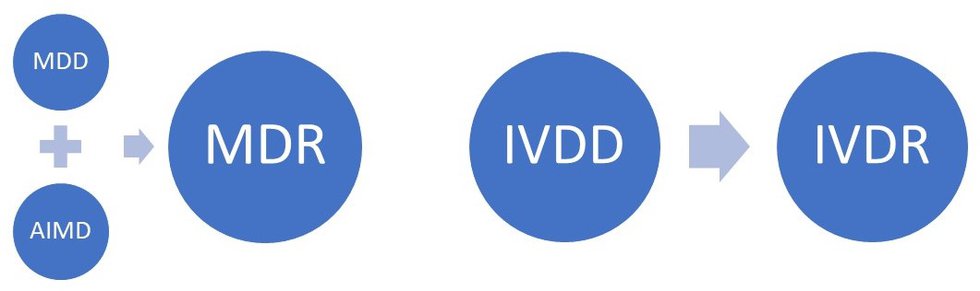
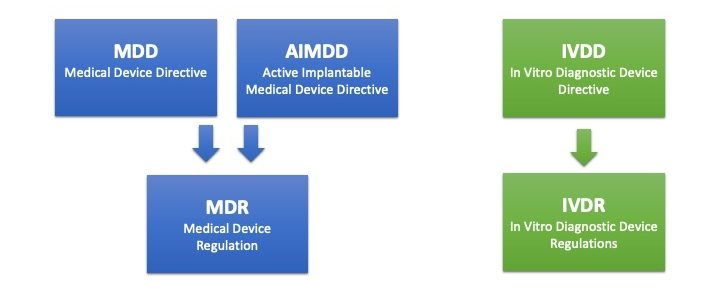
Reglamentos: Publicada la propuesta de reglamento modificando MDR e IVDR con la ampliación de los periodos transitorios

EU Finalizes New Medical Device Regulations (MDR) which update the regulatory framework for the marketing of devices and IVDs in Europe – Catchtrial