
AIGF Partners With Arthur D. Little To Reinforce Its Self-Regulation Process - All India Gaming Federation

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation: Molecular Therapy - Methods & Clinical Development
▻B REGULATION (EU) No 536/2014 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 April 2014 on clinical trials on medicinal
Guide to Clinical Trials Regulation (EU) No. 536/2014 Pilot Project - Ireland Voluntary Pilot Project for the Processing of App

FEAD concerned over Parliament's position on Waste Shipment Regulation revision: circular economy requires both demand and shipping options - FEAD - European Waste Management Association
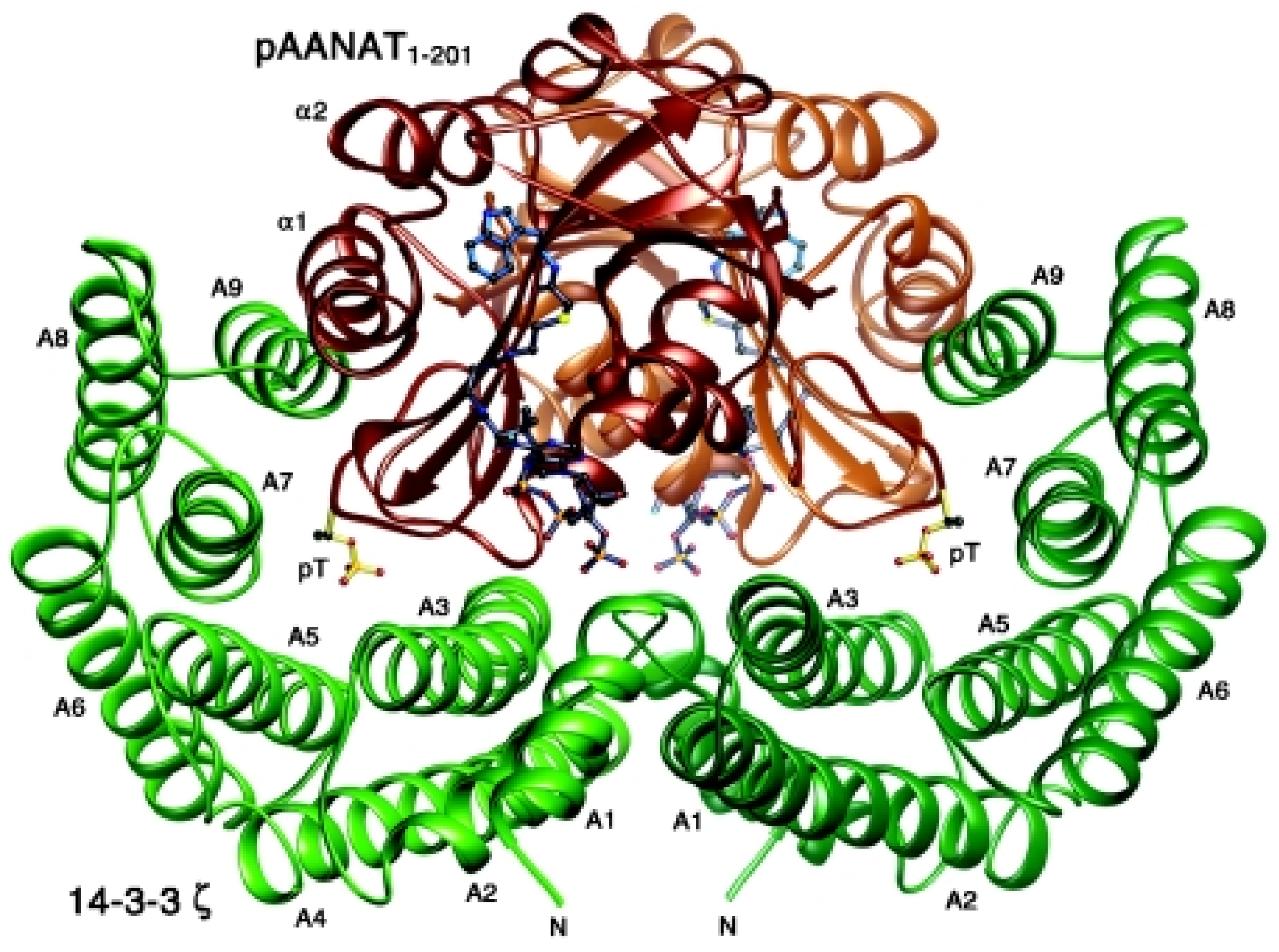
Endocrines | Free Full-Text | Interactions between 14-3-3 Proteins and Actin Cytoskeleton and Its Regulation by microRNAs and Long Non-Coding RNAs in Cancer

EU Cooperation of Member States in Safety Assessment of Clinical Trials published today in Official Journal

PDF) Value of substitute consent and autonomy of research participants in Regulation (EU) No 536/2014










