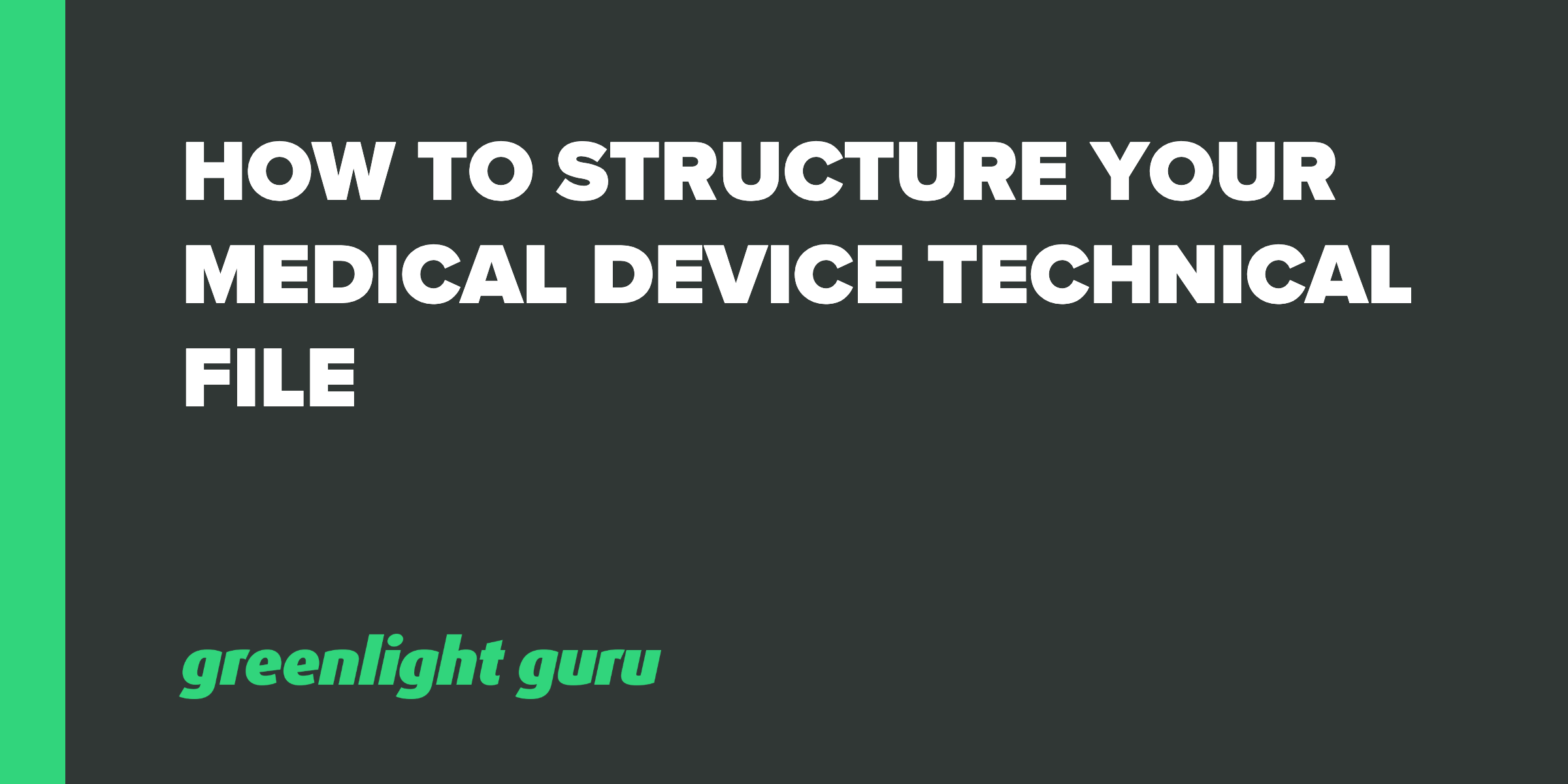
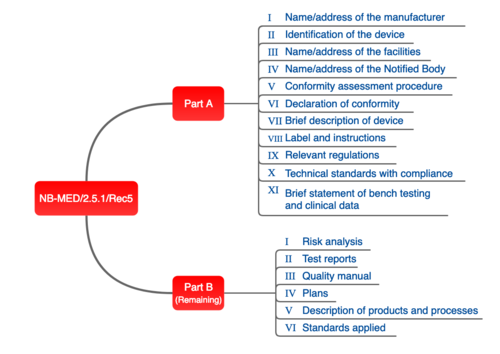
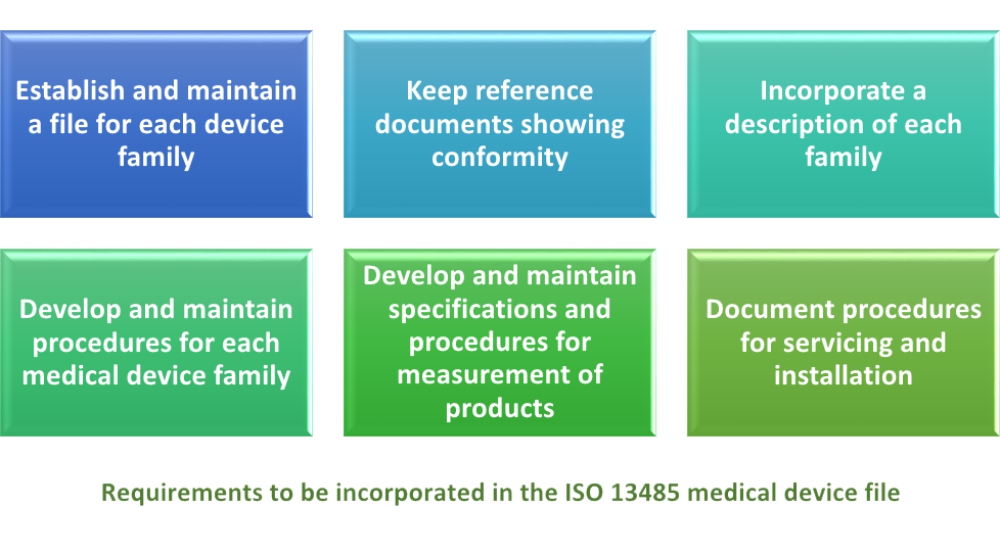
Ce Technical File (Helpful For Machine, Pressure, Emc, Lvd And Medical Device) in Navrangpura, Ahmedabad - Global Quality Kit

Device Master File - Appendix-II - Medical Device-Format | PDF | Verification And Validation | Medical Device

Documentation Services - Technical Documentation for Medical Devices, Technical File, Device Master File Service Provider from Unnao
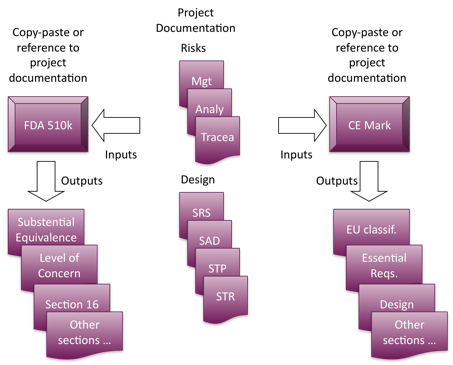


![Technical File Template [ISO 13485 templates] Technical File Template [ISO 13485 templates]](https://advisera.com/wp-content/uploads//sites/14/2021/08/24.1_Technical_File_Template_Integrated_Preview_EN.png)

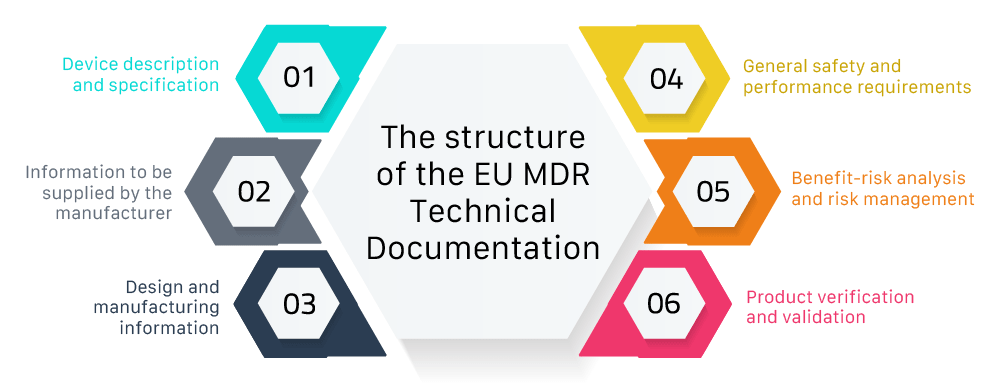
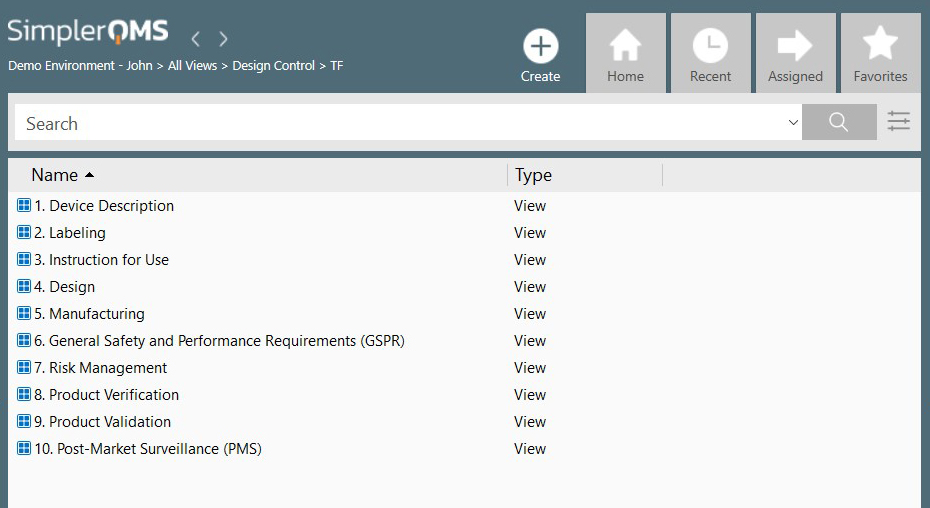
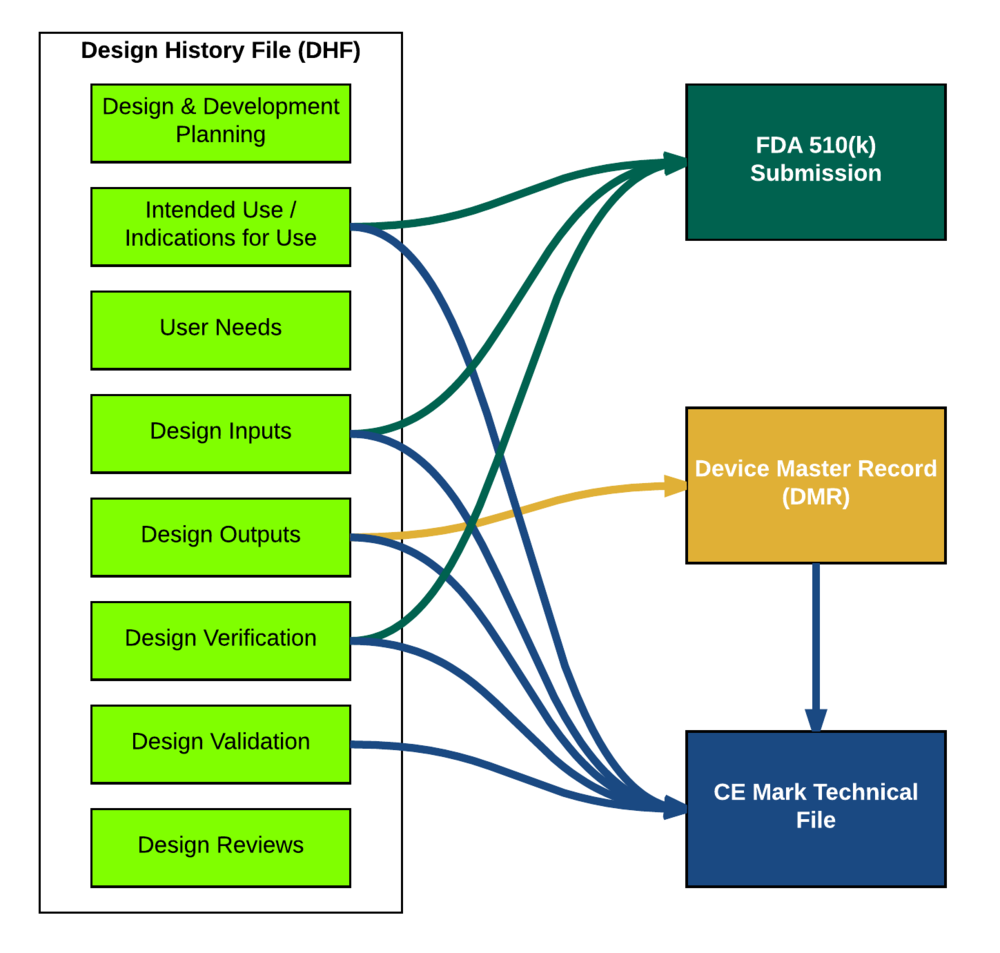

.webp)