1 a Schematic representation for Beer-Lambert law for the measurement... | Download Scientific Diagram
![Integrated Rate Law Expresses the reactant concentrations as a function of time. aA → products Kinetics are first order in [A], and the rate law is Rate. - ppt download Integrated Rate Law Expresses the reactant concentrations as a function of time. aA → products Kinetics are first order in [A], and the rate law is Rate. - ppt download](https://slideplayer.com/14797333/90/images/slide_1.jpg)
Integrated Rate Law Expresses the reactant concentrations as a function of time. aA → products Kinetics are first order in [A], and the rate law is Rate. - ppt download

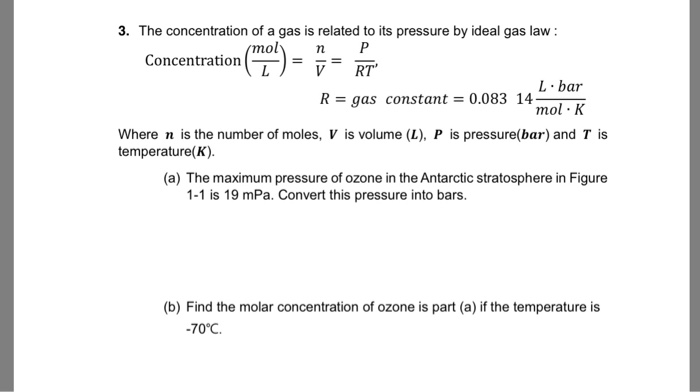
SOLVED: The concentration of a gas is related to its pressure by the ideal gas law: Concentration mol L = n/ V = P/ RT , R = gas constant = 0.08314
Henry's law constant of CO2 in water at 298 K is 5/3 K bar. Determine its concentration (mole fraction) in rain if CO2 is 1
![For reaction A → B , the rate law expression is - d[A]dT = k[A]^1/2 . If initial concentration of [A] is [A]0 then, which of the following statement is incorrect? For reaction A → B , the rate law expression is - d[A]dT = k[A]^1/2 . If initial concentration of [A] is [A]0 then, which of the following statement is incorrect?](https://dwes9vv9u0550.cloudfront.net/images/7410317/0b743bb0-acfd-4fcf-a3d4-3231d770d471.jpg)
For reaction A → B , the rate law expression is - d[A]dT = k[A]^1/2 . If initial concentration of [A] is [A]0 then, which of the following statement is incorrect?

For the reaction A + B rarrC + D , doubling the concentration of both the reactants increases the reaction rate by 8 times and doubling the concentration of only B simply
![SOLVED: 1. A rate law is given as: rate = k[A][B]2 If the units for the concentration terms are M (mol/L), and the rate is given in terms of M/s, what are SOLVED: 1. A rate law is given as: rate = k[A][B]2 If the units for the concentration terms are M (mol/L), and the rate is given in terms of M/s, what are](https://cdn.numerade.com/ask_previews/32d19e93-8a91-4fe8-a37e-cc016681e241_large.jpg)










:max_bytes(150000):strip_icc()/beers-law-definition-and-equation-608172_FINAL-20ddc4fef437472db0a0ebe395770c76.png)