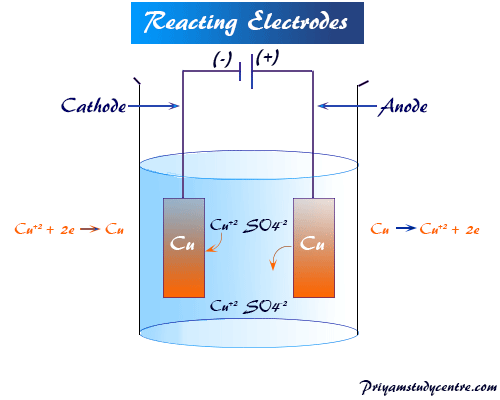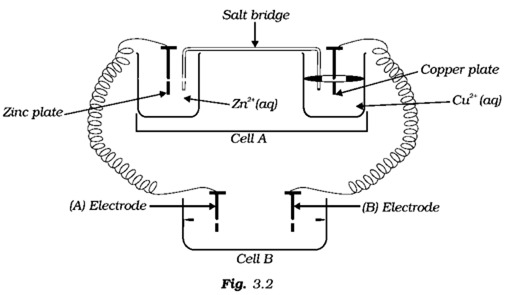
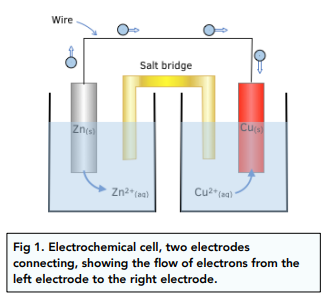
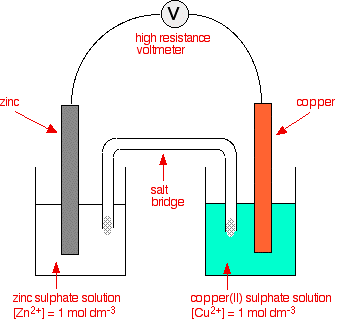
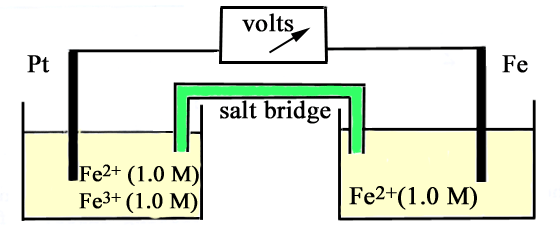
Consider the Fig. 3.2 and answer the following questions. (i) Cell 'A' has E Cell = 2V and Cell 'B' has E Cell = 1.1V which of the two cells 'A' or '

Electrode Reactions of Elemental (White) Phosphorus and Phosphane PH3 - Yakhvarov - 2013 - European Journal of Inorganic Chemistry - Wiley Online Library
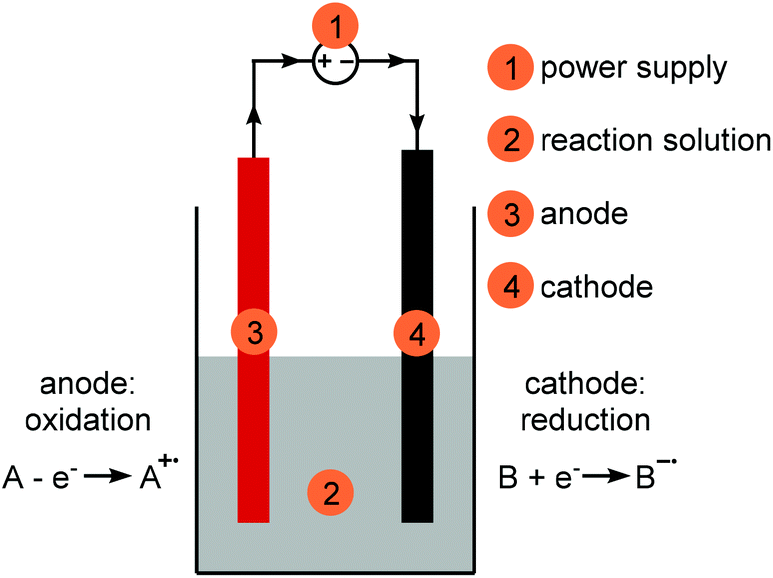
Making electrochemistry easily accessible to the synthetic chemist - Green Chemistry (RSC Publishing) DOI:10.1039/D0GC01247E

Surface Electrochemistry of Carbon Electrodes and Faradaic Reactions in Capacitive Deionization | Environmental Science & Technology

pH Overpotential for Unveiling the pH Gradient Effect of H+/OH− Transport in Electrode Reaction Kinetics | CCS Chem

Prediction of the Current Density at an Electrode at Which Multiple Electrode Reactions Occur under Potentiostatic Control | Semantic Scholar

Discrimination of Inner- and Outer-Sphere Electrode Reactions by Cyclic Voltammetry Experiments | Journal of Chemical Education

Counter Electrode Reactions—Important Stumbling Blocks on the Way to a Working Electro‐organic Synthesis - Klein - 2022 - Angewandte Chemie International Edition - Wiley Online Library
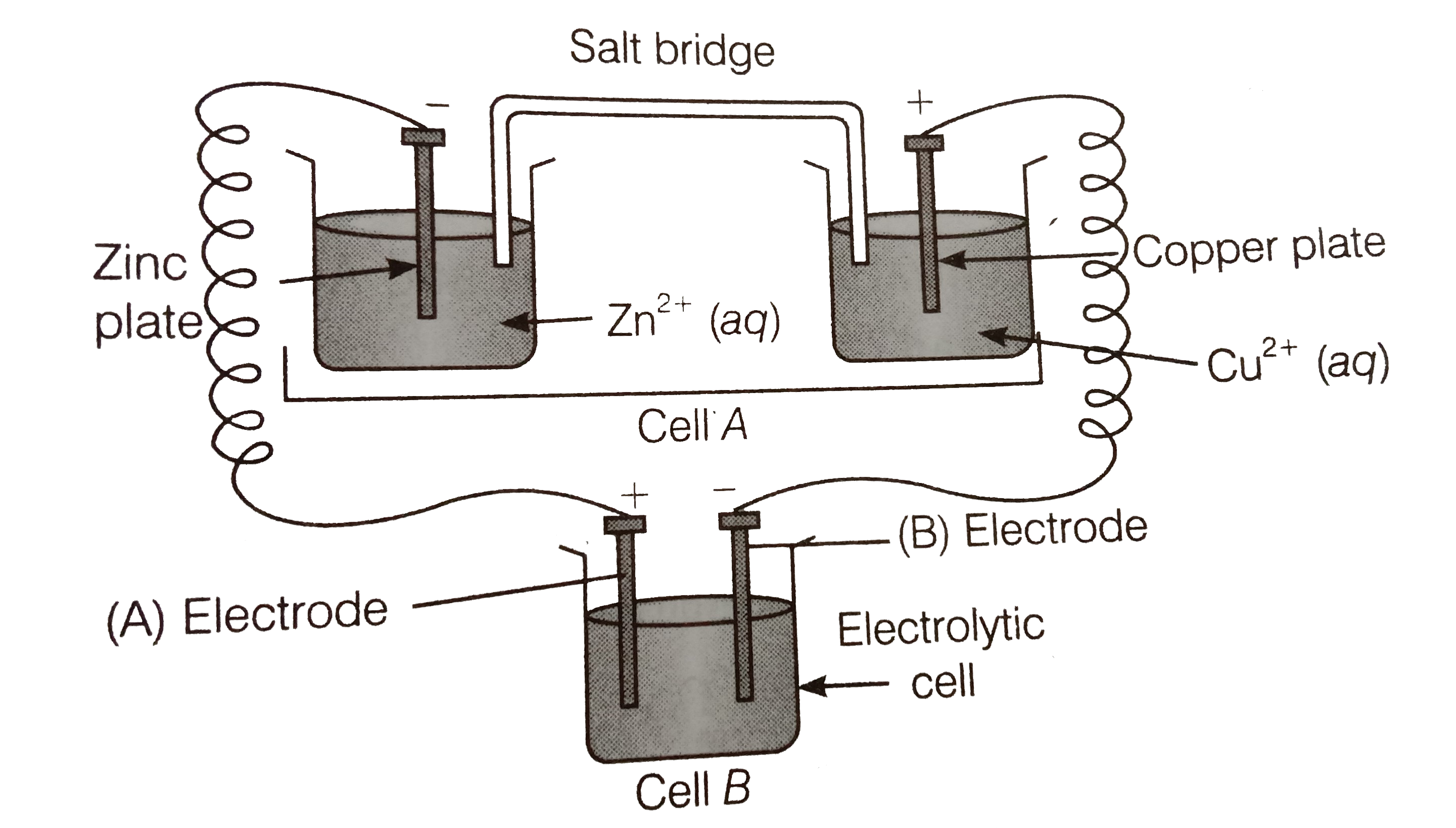
Consider the figure and answer the following question. (i) Cell 'A' h as E("cell")=2V and Cell 'B' has E("cell")=1.1V which of the two cell 'A' or 'B' will act as an electrolytic



![10 Pathway of a general electrode reaction [26]. | Download Scientific Diagram 10 Pathway of a general electrode reaction [26]. | Download Scientific Diagram](https://www.researchgate.net/publication/35398081/figure/fig6/AS:646793398349827@1531218971435/Pathway-of-a-general-electrode-reaction-26.png)


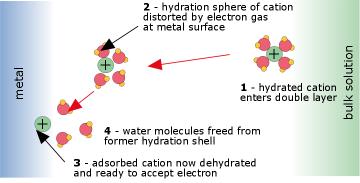
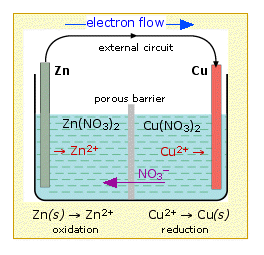
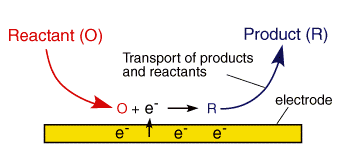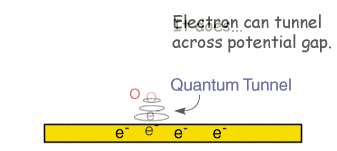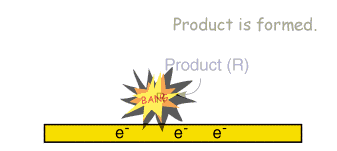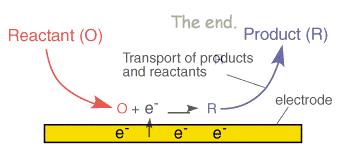
![Electrode potentials [SubsTech] Electrode potentials [SubsTech]](https://www.substech.com/dokuwiki/lib/exe/fetch.php?w=&h=&cache=cache&media=galvanic_cell.png)