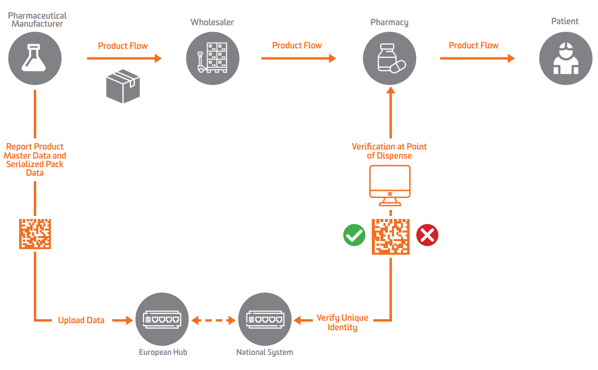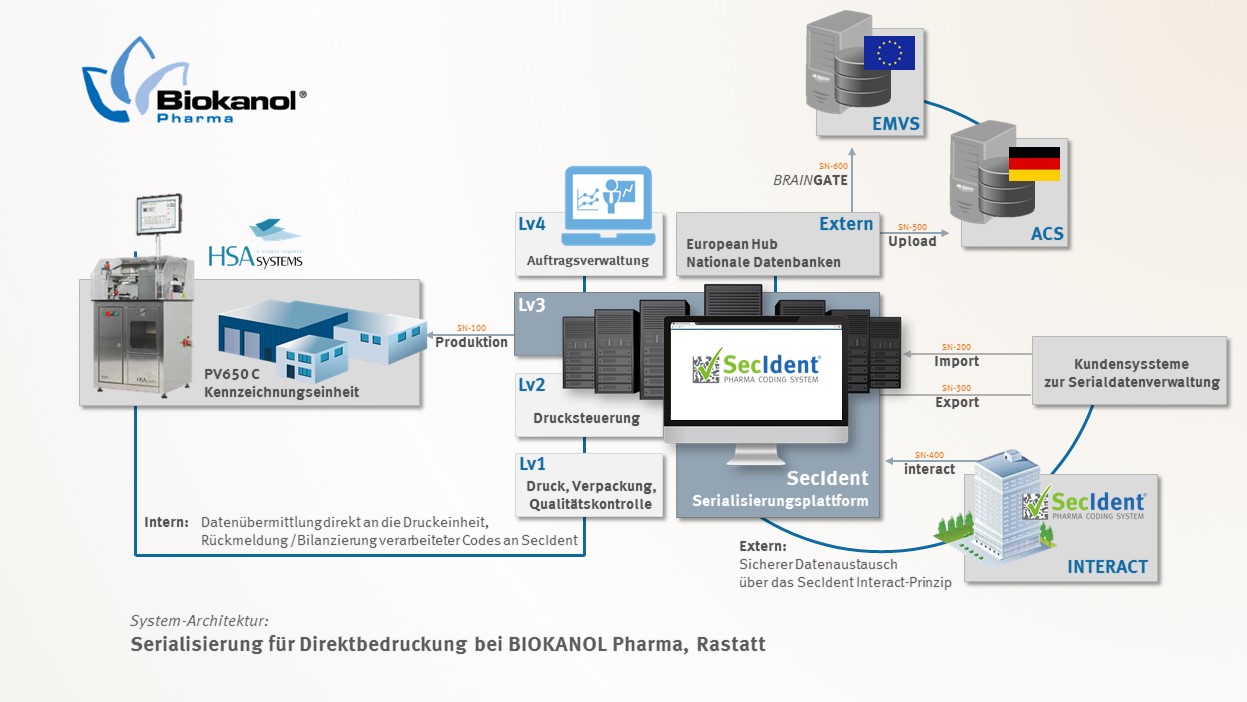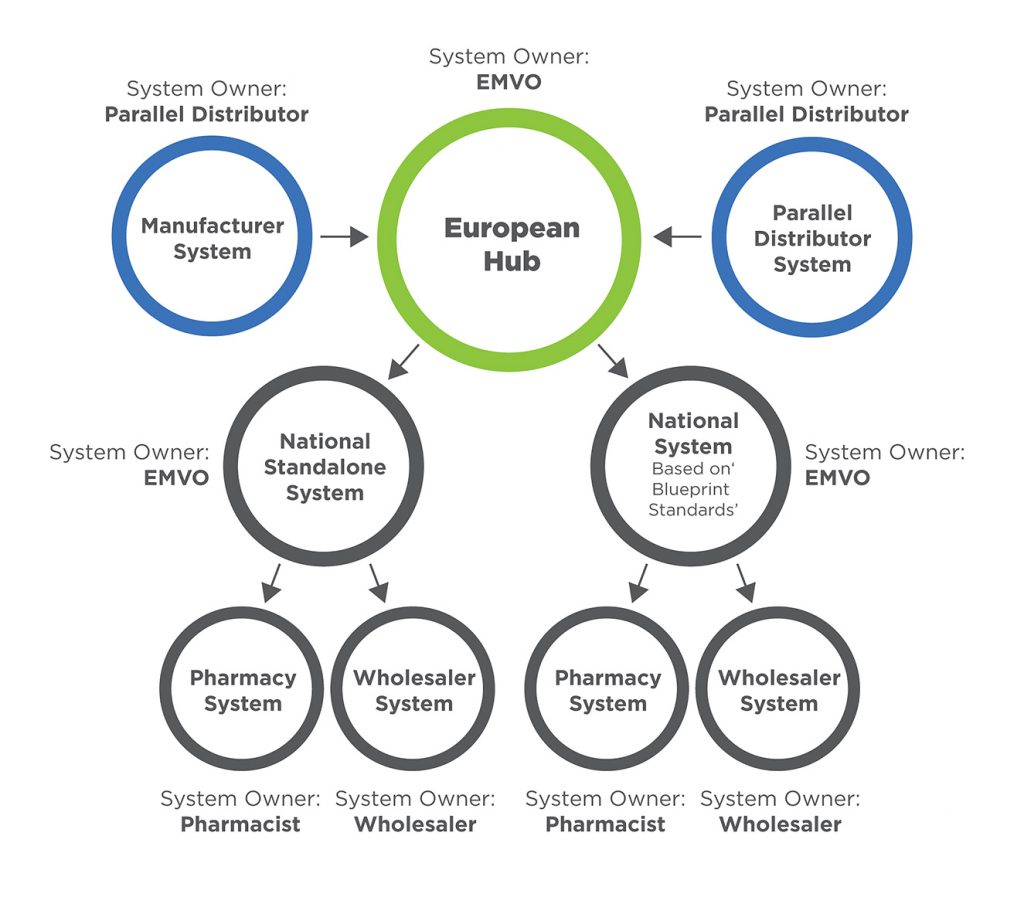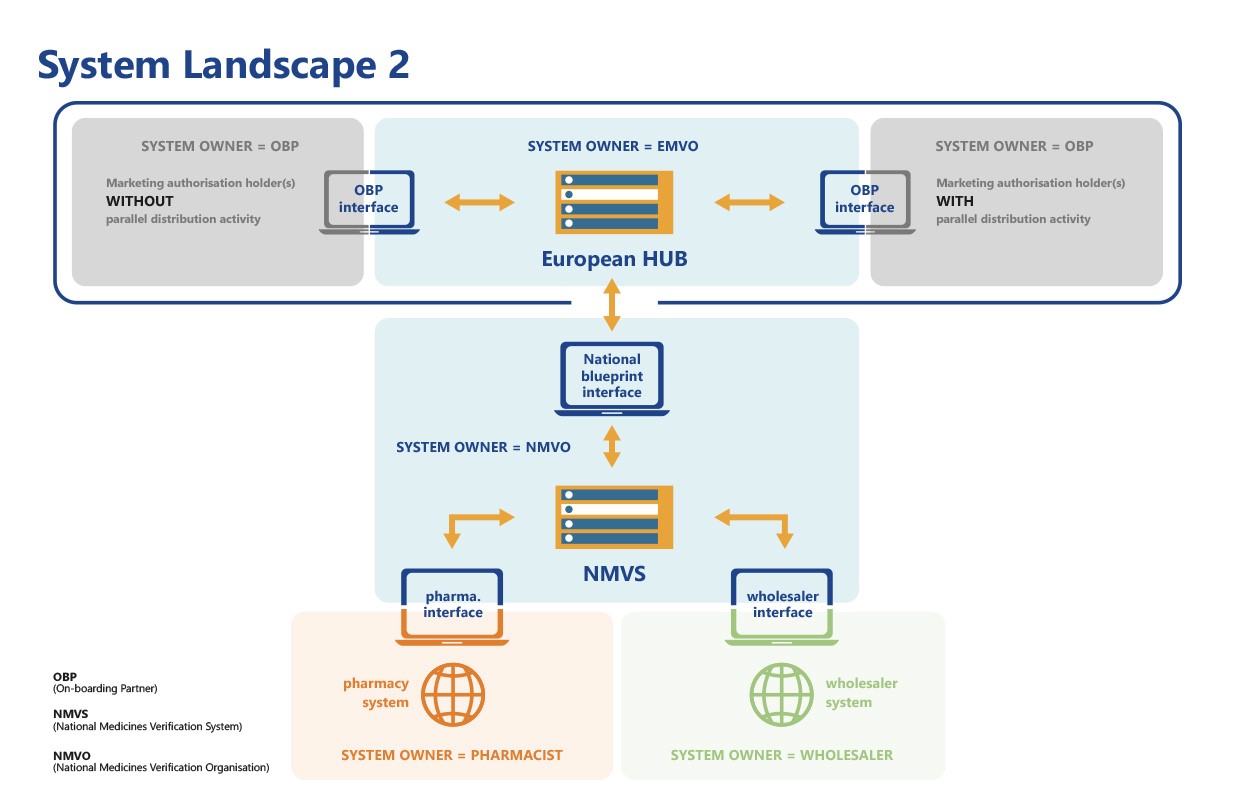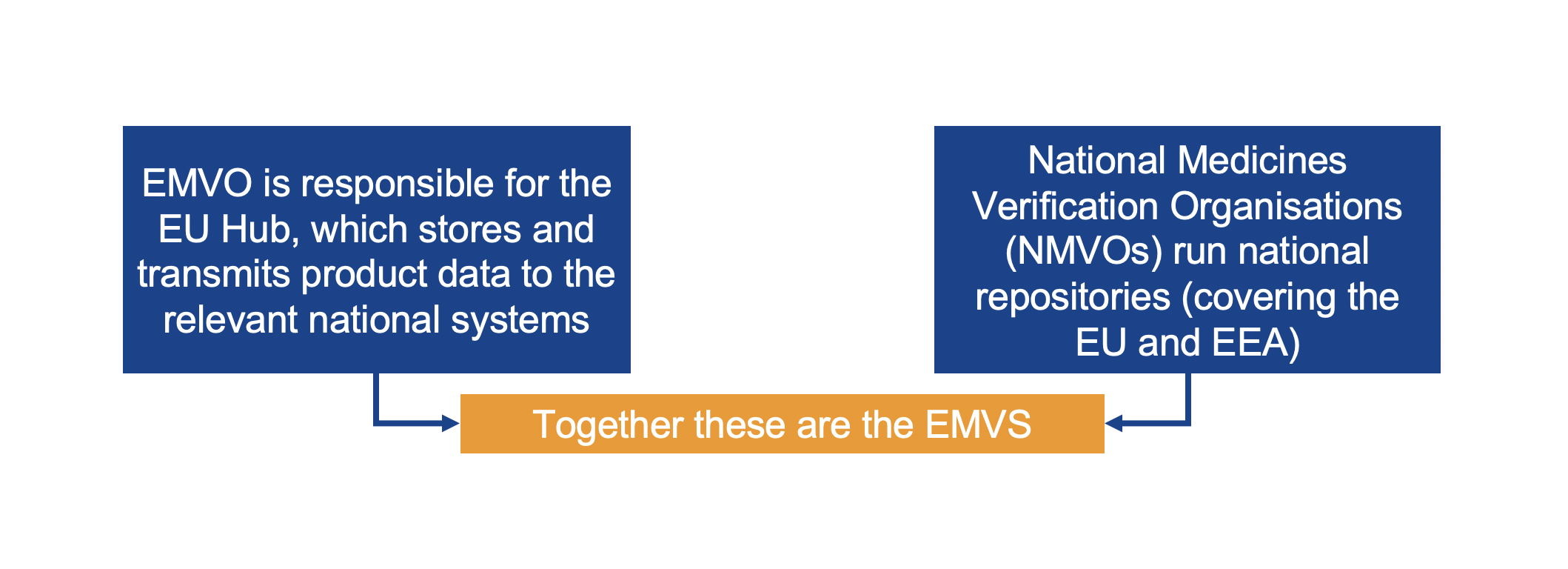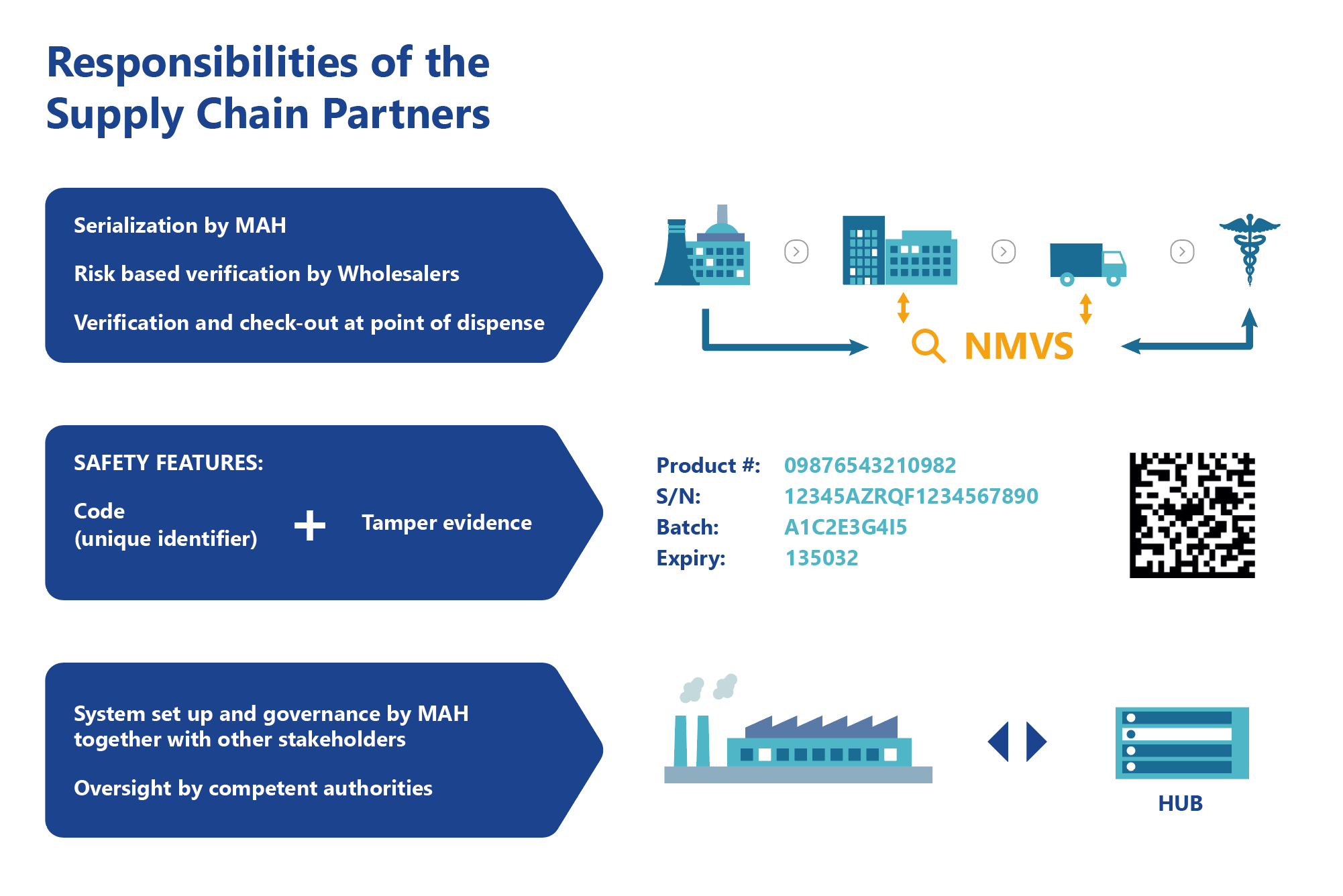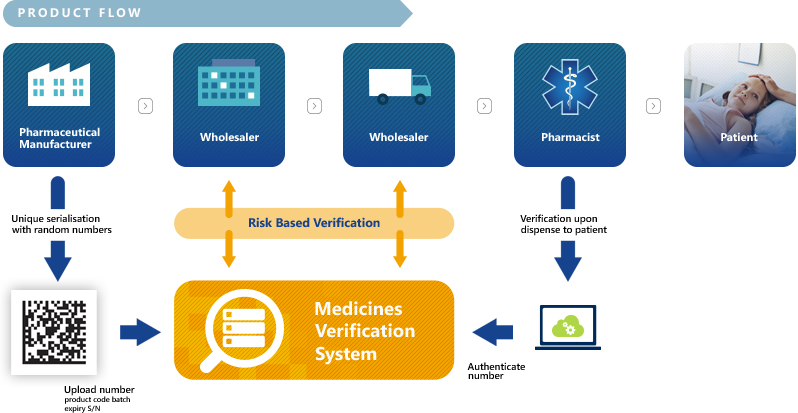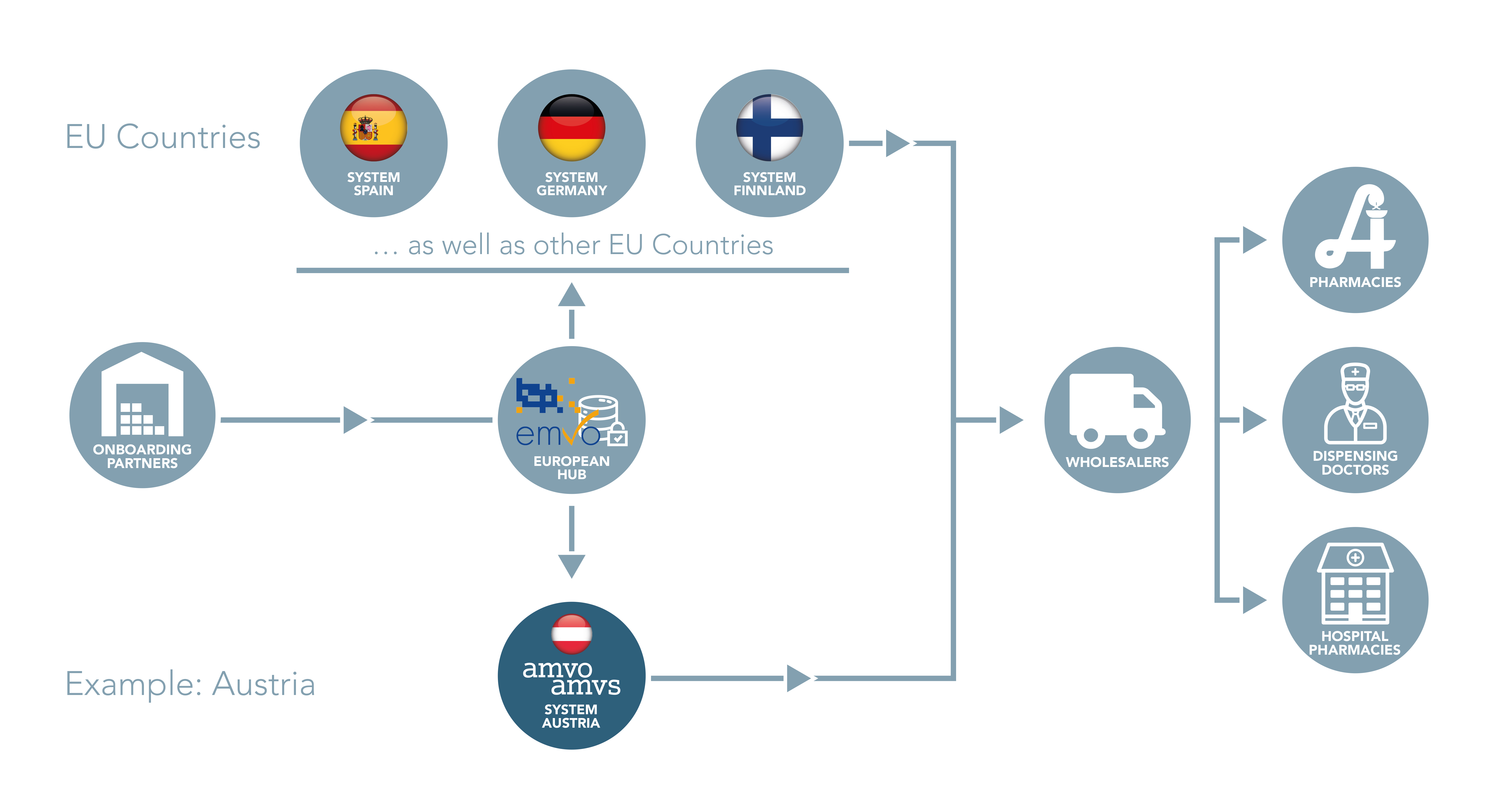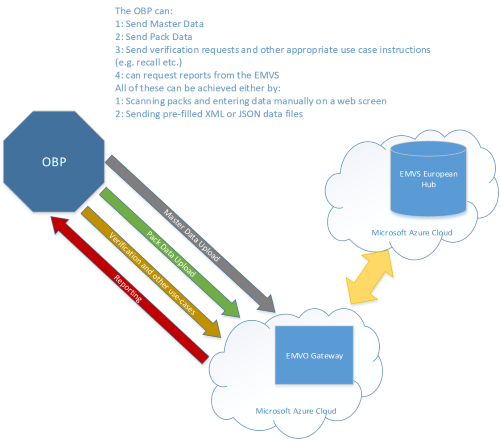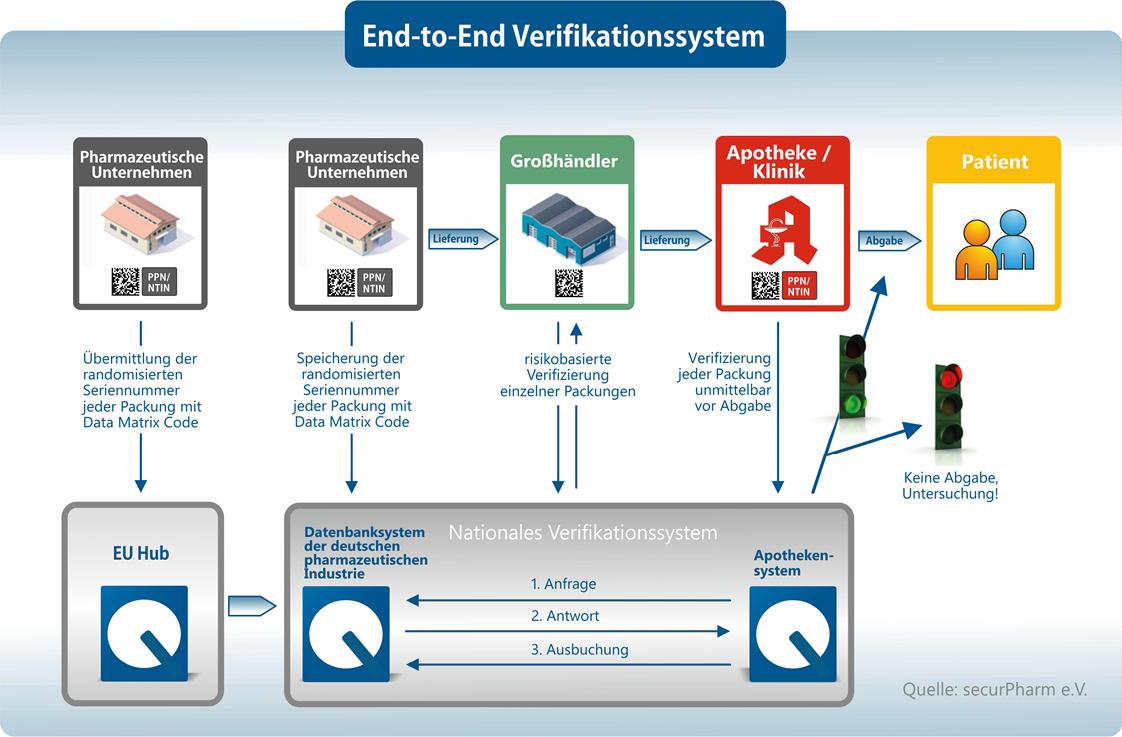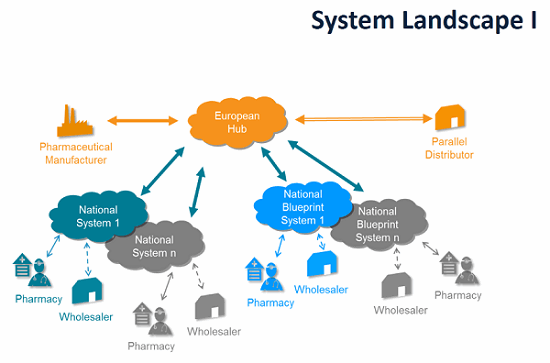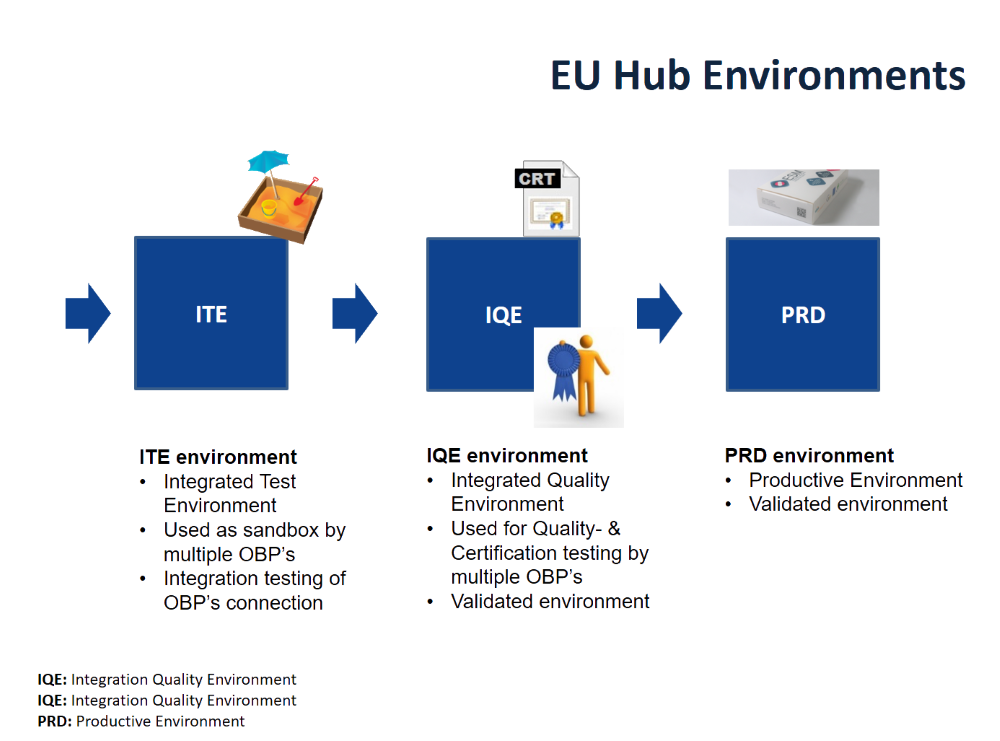
EMVO certifies Arvato Systems serialization solution Arvato CSDB for EU Hub, Arvato Systems GmbH, Press release - PresseBox
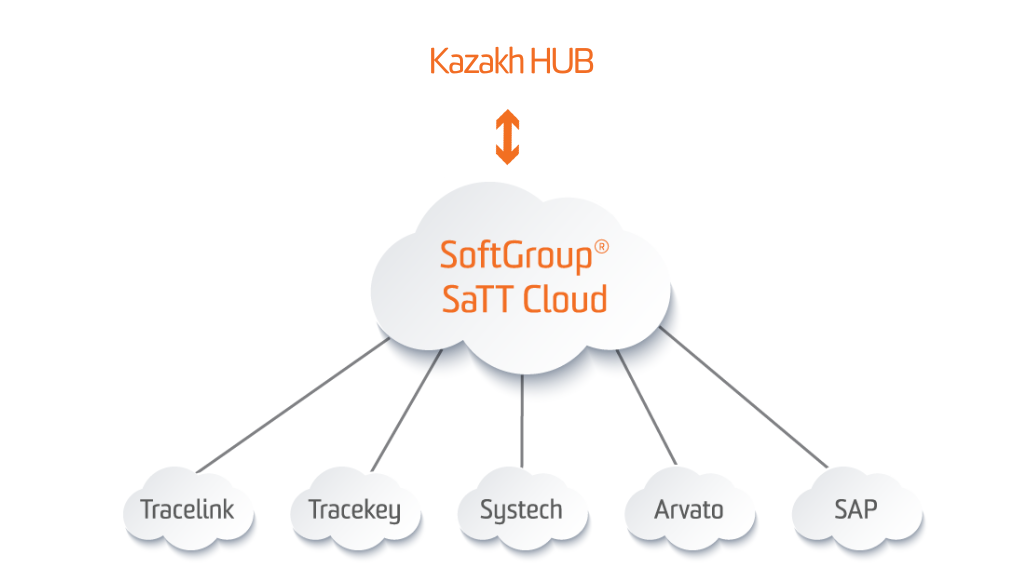
Cloud services: Regulatory Compliance Software - Pharma Serialization, Aggregation and Track and Trace Software by SoftGroup